Weiter: Korrekturen Oben: Vorlesungsskript PHYS2100 Grundlagen der Zurück: Umrechnungen: Harmonischer Oszillator Skript: PDF-Datei Übungen: Blätter
(Siehe Handbook of Chemistry and Physics [Wea80, pp. D-120])
Die folgenden Funktionen werden betrachtet:
Aus den folgenden Tabellen können die ersten Ableitungen von ![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() und
und ![]() als
Funktion von
als
Funktion von
![]() ,
,
![]() und
und
![]() berechnet werden. Diese Grössen können gemessen werden:
berechnet werden. Diese Grössen können gemessen werden:
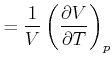 |
isobare Volumenkompressibilität | (K..786) | |
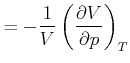 |
isotherme Kompressibilität | (K..787) | |
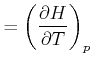 |
molare Wärmekapazität bei konstantem Druck | (K..788) |
Beispiel:
Wir wollen
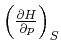 als Funktion von
als Funktion von ![]() ,
, ![]() und
und ![]() berechnen.
berechnen.
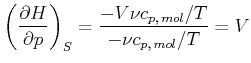 |
Wir wollen
![]() als Funktion von
als Funktion von ![]() ,
, ![]() und
und ![]() berechnen.
berechnen.
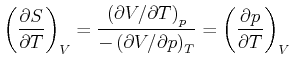 |
| ||||||||||||||||||||||||||||||
|
|
|
|
|
|
Othmar Marti